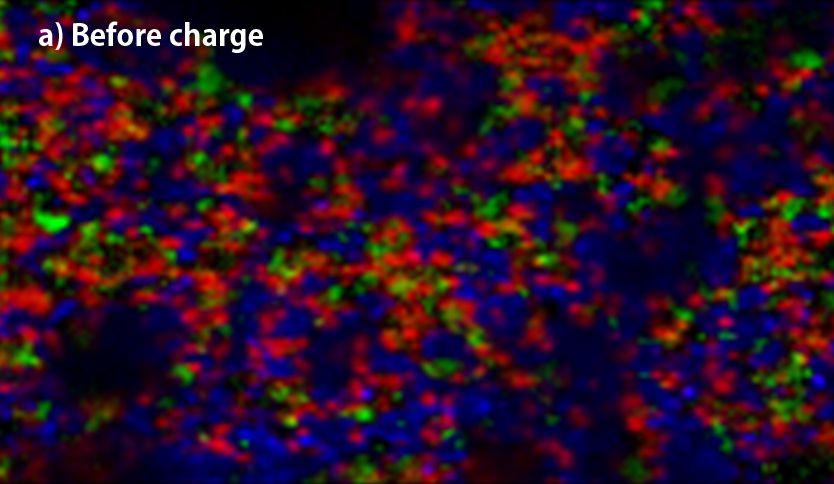
▲Raman-imaging comparison between before(a) and after charge(b) of anode.
(red; crystalline-Si, yellow; amorphous-Si, blue; graphite, green; Kedjen black)
| Wavelength | 532 nm |
| Obj. lens | 100x (NA=0.85) |
| Number of spectra | 40,000 (400×100) |
| Measurement time | 35 min |
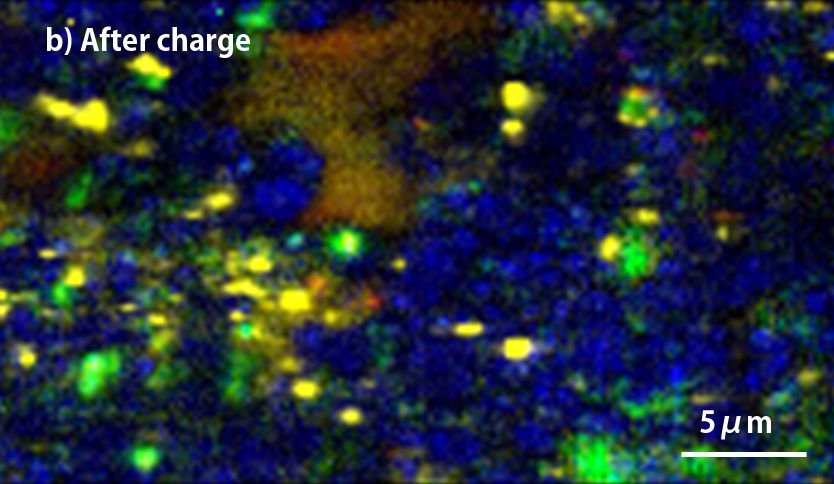
Silicon that absorbs more Li+ than graphite is the leading material for high capacity anode in Li-ion batteries(LIB), but Silicon crystal has the disadvantage that it is almost broken throughout charge-discharge because of its drastic change of volume. The effective ways to prevent the silicon crystal damage are miniaturization and finely dispersion of silicon crystal with other ingredients of anode. Using sodium polyacrylate binder achieve the finely dispersion and it could be confirmed with the raman-imaging. Especially, it can be observed that the silicon particles are dispersed less than 1μm.
The above two figures display raman-imaging comparison between before and after charge of graphite-Sipolyacrylate anode. The charged anode was taken out from the cell in a glovebox, and then set it into LIBcell (closed vessel). This makes it possible to measure raman-imaging keeping the state of charge under the air. It could be confirmed that almost crystalline-Si was changed to amorphous shape because of intercalation of Li+. On the other hand, the graphite-Si-PVdF anode, which was not well dispersed, was observed to have the major change of form in the raman spectrum at G-band of carbon compared to silicon. Raman-imaging also can confirm the difference of electrical characteristic caused by dispersion form of ingredients of anode.
Visualize the fine dispersion of active materials with high spatial resolution
Silicon, which can store more lithium than graphite, is a promising negative electrode material for high capacity lithium ion batteries. However, the silicon crystal collapses due to the large volume change before and after charge and discharge, and there are problems in the cycle characteristics. It is effective to finely disperse micronized silicon as one of the means to prevent the collapse of silicon crystals due to volume change, but using sodium polyacrylate (PANa) as a binder allows each component to be dispersed homogeneously It was confirmed by Raman imaging (see below). In particular, silicon appears finely dispersed to less than 1 μm. On the other hand, in the electrode plate using PVdF as the binder, it can be seen that the mixture of graphite and ketjen black is biased compared to the case of using PANa.
| Excitation wavelength | 532 nm |
| Obj. lens | 100x (NA=0.90) |
| Number of spectra | 22,500 |
| Measurement time | 27 min |
Evaluate lithium storage condition from Raman spectrum
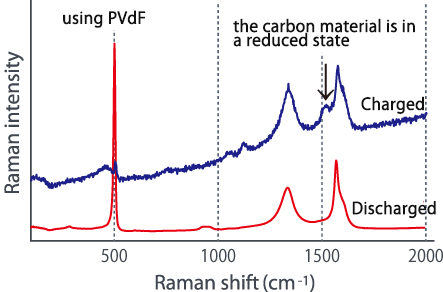
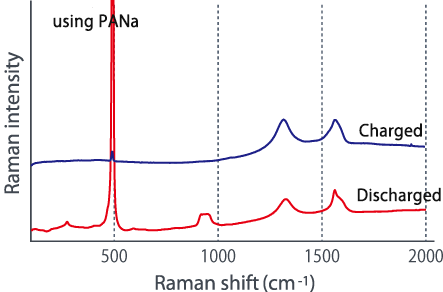
Comparing the Raman spectra before and after charging using these two types of electrode plates in different dispersion states, a large change is seen in the G-band of the carbon material, and lithium is absorbed in the carbon material. It was suggested that it might not be efficiently contained in silicon (see below). In this way, we were able to confirm the difference in the characteristics of lithium ion batteries due to the dispersed state from the Raman spectrum change.
■Raman spectrum change before and after charging
※ This sample was provided by Komaba Laboratory, Tokyo University of Science.
Nanophoton product
→充放電in-situラマン測定用セル LIBcell charge
→不活性雰囲気ラマン測定用密閉容器 LIBcell