Email Magazine
Free research for adults (8/18/2021)
Continuing from last time, we will use the cooling and heating stage to make measurements. What we have set our eyes on is mayonnaise. When mayonnaise is frozen and melted, the water and oil separate. What does it look like when we follow this phenomenon with a Raman microscope? Again, Mr. Katsuhito Aoki of the Nanophoton Tokyo Showroom measured it for us. (Email Newsletter editor-in-chief / freelance writer Takeshi Nemoto)

The main ingredients of mayonnaise are cooking oil, vinegar, and eggs. Oil and water (vinegar) normally do not mix and separate, but thanks to the egg component, the fine particles of oil are dispersed in the water. In other words, it is emulsified.
However, once you freeze it and bring it back to room temperature, the water and oil will separate. I’m sure many of you have experienced this. Once they are separated, they will not return to their original state.
In order to measure the separation with a Raman microscope, the sample needs to be lowered to freezer temperature. Again, we borrowed a “Peltier-type cooling and heating stage for microscopes” from Japan High Tech Corporation, which sells specialized equipment. It is possible to lower the temperature to minus 40 degrees Celsius by electronic cooling without using liquid nitrogen. We attached it to the laser Raman microscope RAMANtouch and measured it!

The mayonnaise is not commercially available, but handmade by Mr. Aoki. Raman images were measured in the following order: room temperature (25°C), -20°C, -15°C, -10°C, -5°C, 0°C, 5°C, and room temperature (25°C). When the temperature was changed, it was changed at a rate of 10°C/minute, and the temperature was kept constant during the measurement.
At room temperature (25°C), there was water around the spherical lipid (edible oil), indicating that it was in an emulsified state. When it was cooled to -20°C, the water froze.
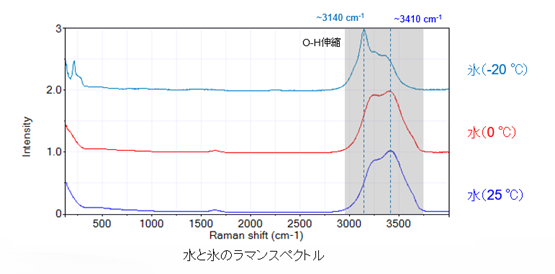
Here is a review of the previous section. When we measure the Raman spectra of water and ice, the position of the strongest peak changes, so we can distinguish between water and ice with the Raman microscope.
Now, let’s increase the temperature by another 5°C and take measurements.
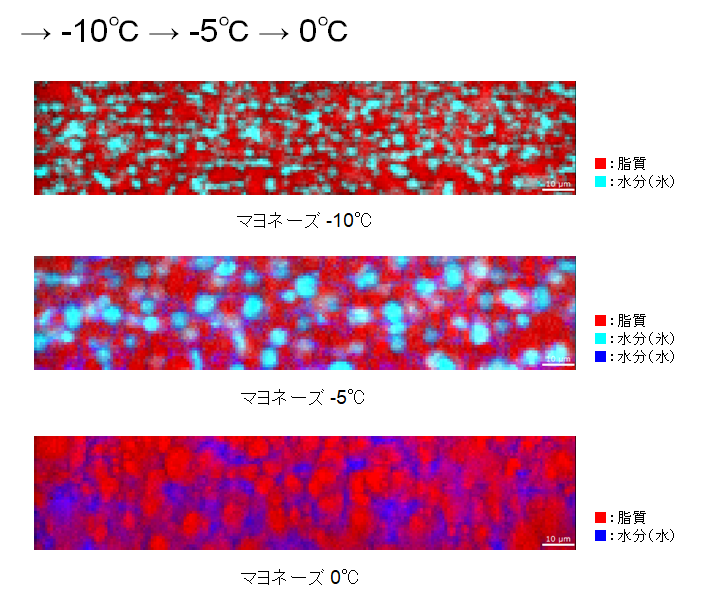
When the temperature was raised to -10°C, the oil grains started to lose their shape. You can see that there is ice in between the oil.
-5°C, the ice particles seem to be growing larger. The water is distributed among the oil. When the temperature was further raised to 0°C, the ice grains melted and became water.
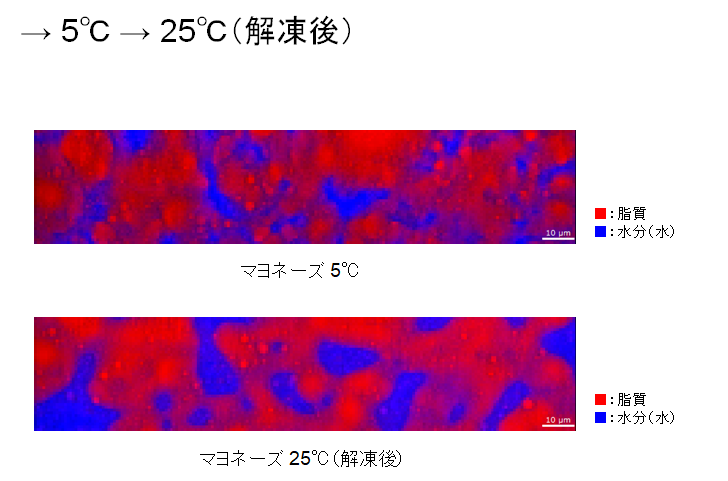
When the temperature is further increased to 5°C and 25°C, the oil and water become large chunks respectively. They are now completely separated.
Let’s look at the Raman spectrum at -5°C.

Each component is colored according to the area of the peak indicated by the arrow (↓). Lipids are around 2854 cm-1 , ice is around 3150 cm-1 , and water is around 3190-3520 cm-1 .
The optical microscope image of the same area looks like this.

The optical microscope image does not reveal what the visible grains are, but the Raman microscopy clearly identifies them as ice grains, not oil.
In this article, we actually measured how mayonnaise looks under a Raman microscope when it is frozen and then melted. But why does it separate when frozen? If it is possible, I will definitely try to find out.